Thursday, July 14, was a notable day at ARA. The team at the ARA Theranostics Center administered the first dose in Central Texas of Lu-177 PSMA (Pluvicto) to a prostate cancer patient. With this treatment begins an era at ARA of innovative prostate cancer therapy that is minimally invasive and targeted to treat just the cancer cells. Prostate cancer is the most commonly diagnosed cancer among men in Texas: about one in eight men will be diagnosed during his lifetime. While most cases are survivable, it is estimated that 2,260 men will die of prostate cancer in Texas this year. This effective, innovative treatment is great news for Central Texas patients.
The FDA approved Lu-177 PSMA-617 in March of 2022 for treatment in certain men with metastatic prostate cancer. Known by the brand name Pluvicto™, Lu-177 PSMA-617 is the first of a new class of PSMA-based radiopharmaceuticals that use selective targeting to eliminate prostate cancer cells, providing a treatment option for men whose prostate cancer has progressed despite having received traditional therapies.
Download ARA’s patient flyer on Lu-177 PSMA Pluvicto cancer treatment
HOW DOES PLUVICTO WORK?
Pluvicto™ has a strong attraction to prostate cancer cells but does not affect other healthy prostate cells. After being given by intravenous injection, it circulates through the body locating and attaching itself to prostate cancer cells wherever they are — even pockets of cancer that are not visible to the naked eye or conventional imaging. It then eliminates those cells with minute amounts of radiation, leaving the surrounding tissues virtually untouched.
The treatment course for patients entails six visits to the ARA Theranostics Center, each six weeks apart. The infusion takes 30 minutes to administer, and each visit, including associated care, takes between two to three hours.
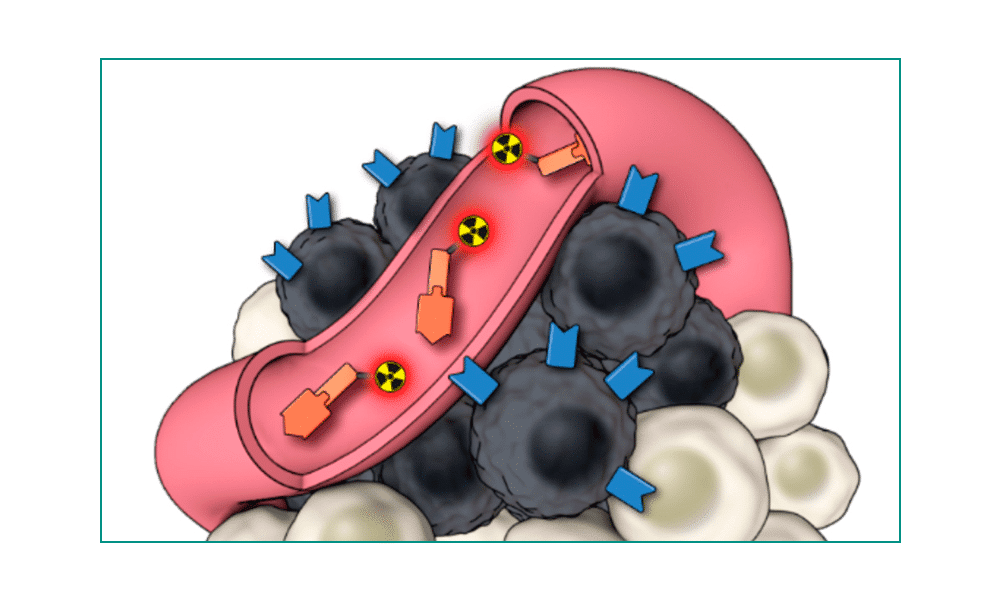
1) Pluvicto is infused through an IV. 2) Pluvicto seeks out prostate cells (in black) anywhere they have metastasized in the body. 3) Pluvicto attaches to the prostate cancer and delivers a microscopic amount of radiation to the cancer cells. 4) The radiation kills the cancer cells, leaving surrounding healthy tissue (in white) unharmed.
WHO IS A CANDIDATE FOR PLUVICTO?
Men with metastatic, castrate-resistant prostate carcinoma (mCRPC) are potential candidates for Pluvicto. In other words, these are men whose prostate cancer has progressed despite prior therapies such as surgical resection, radiation therapy, androgen receptor pathway inhibition, and/or taxane-based chemotherapy.
PET/CT imaging with PSMA is required to confirm that the metastases will accumulate PSMA, and therefore respond to Pluvicto.
Guidelines may change in the future. Anyone interested is encouraged to discuss Pluvicto with their cancer care provider. Inquiries can be directed to Alex DiFonzo at (512) 519-3456, ext. 2351, or the theranostics team at theranostics@ausrad.com.
INSURANCE COVERAGE FOR PLUVICTO
Pluvicto is currently covered by Medicare for some situations, but not others. Uniform coverage for all situations is expected by the end of the year. Private insurance coverage tends to follow Medicare coverage. Patients are encouraged to check with our theranostics team for answers about eligibility. In the meantime, Pluvicto™ is available for patients on a self-pay basis.
WHAT IS THERANOSTICS?
Theranostics is a medical field dedicated to the diagnosis and treatment of cancer through the use of targeted radiopharmaceuticals. These are medicines that have a strong attraction to cancer cells. Different radiopharmaceuticals can target different types of cancers. Radiopharmaceuticals are typically infused into the bloodstream, after which they circulate through the body locating and attaching to cancer cells wherever they are — even pockets of cancer that are not visible on conventional imaging. ARA has specialized scanners that can detect the radiopharmaceuticals and show where the cancers are located in the body.
In certain instances, the radiopharmaceutical that is used for imaging can be modified to treat cancer by delivering minute amounts of radiation directly to the cancer cells, eliminating the cancer while leaving the surrounding tissues virtually untouched. This is called radiopharmaceutical therapy.
ABOUT THE ARA THERANOSTICS TEAM
The ARA theranostics team includes six molecular radiologists who are all double-boarded in diagnostic radiology and nuclear medicine, plus highly-trained nuclear medicine technologists and a medical support group that works with insurance and scheduling. Our team partners with each patient’s cancer care provider to ascertain and monitor the treatment that is best for each patient.
THE ARA THERANOSTICS CENTER
The Theranostics Center at ARA has been designed at our Midtown location to comfortably and safely administer the radiopharmaceuticals that are used in theranostics treatments. The treatments typically last from 2 to 6 hours depending on the radiopharmaceutical being used and the Center is made to be accommodating for patients during their stay. Wi-Fi and television are available in the infusion room. Patients may also bring a book, computer, or other quiet activity to occupy their time. Snacks and drinks are provided to patients during the treatment period. Lunch is also offered to patients whose treatments last most of the day.
Download a flyer about ARA’s Theranostics Center.
SCHEDULING TREATMENT
A referral from your cancer care provider is required to schedule a consult for your treatment. For more information, please talk to one of our theranostics coordinators at (512) 519-3456, ext. 2351, or email us at theranostics@ausrad.com.